Elon Musk’s neurotechnology company, Neuralink, has recently launched a groundbreaking clinical trial in Great Britain to test its brain-computer interface (BCI) device. This development marks a significant milestone in the advancement of implantable brain chips designed to restore autonomy and improve the quality of life for people suffering from neurological conditions that impair their ability to interact with computers and other electronics.
The clinical trial is set to take place at hospitals in London and Newcastle, following regulatory approval from UK health authorities. The trial will enroll up to seven patients who have severe neurological impairments, such as paralysis from spinal cord injuries or diseases like amyotrophic lateral sclerosis (ALS). The primary objectives are to evaluate the safety, functionality, and real-world usability of Neuralink’s implantable device in controlling digital technology through thought alone.
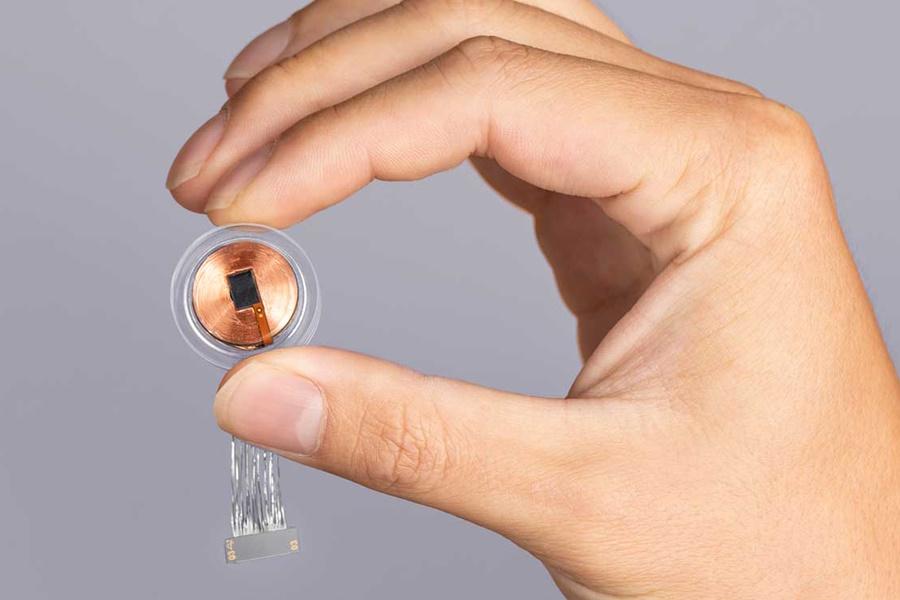
Neuralink’s core technology revolves around a small implant called the N1 device, which is surgically embedded into the skull. The implant contains 1024 electrodes distributed across ultra-thin, flexible threads. These threads interface with neurons in specific brain regions involved in motor control and intention. The implant wirelessly transmits decoded neural signals to an external application that enables users to operate computers, smartphones, or robotic limbs without physical interaction.
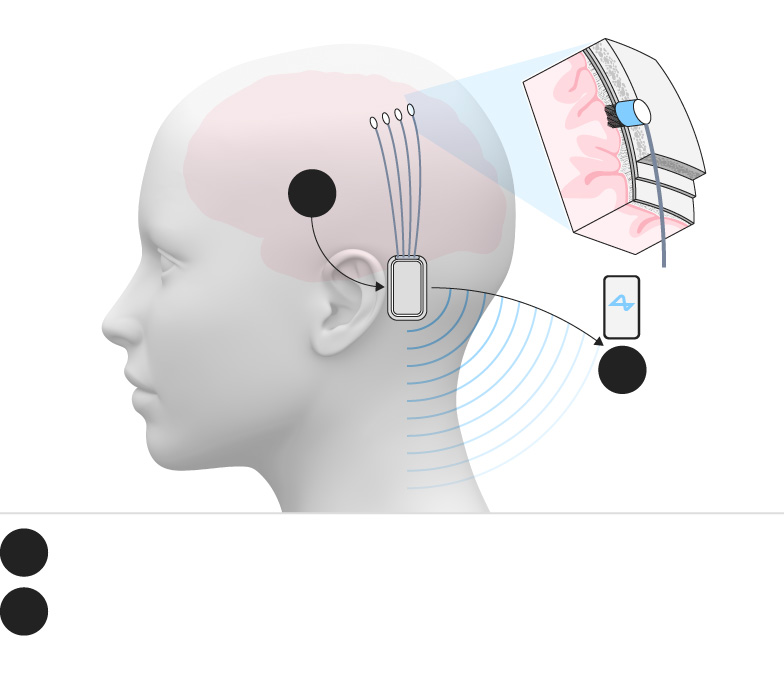
The device’s design is highly sophisticated and includes a wireless rechargeable battery and custom low-power chips that process neural signals in real-time. The threads are inserted using a specially developed surgical robot capable of placing them precisely and safely within delicate brain tissue.
Since receiving initial FDA approval in the United States in 2023, Neuralink has implanted the device in multiple human participants, with the latest data showing nine patients benefiting from the technology. Early results indicate that users can perform tasks such as playing video games, controlling digital devices, and eventually could regain functions lost due to paralysis. The company plans to expand the clinical trial enrollment to 20 to 30 participants by the end of 2025 and pursue further regulatory approvals in multiple countries, including the UK, Canada, and the UAE.
Neuralink’s ambitions extend beyond restoring basic autonomy. Elon Musk envisions a future where brain-computer interfaces enhance human cognitive abilities, enable immersive digital experiences, and even facilitate brain-to-brain communication. This vision aligns with Musk’s broader concerns about the rapid advancement of artificial intelligence, suggesting that merging human brains with AI via devices like Neuralink could be crucial to keeping pace with evolving technologies.
However, Neuralink’s pioneering work has raised a range of ethical and scientific questions. Skeptics express concerns about long-term safety, potential brain tissue damage, mental health implications, privacy risks associated with neural data, and the societal effects of cognitive enhancement technologies. Neuralink asserts its commitment to rigorous safety standards and ethical development as it navigates these challenges.
In June 2025, Neuralink secured $650 million in funding, supporting the company’s extended clinical trials and technology development efforts. This financial boost coincides with the initiation of its global clinical programs and accelerated research into applications such as speech restoration for stroke victims and vision restoration for the blind.
In conclusion, Neuralink’s clinical trial launch in Great Britain signifies a major leap forward in brain implant technology, moving closer to making mind-controlled digital interaction a reality for people with severe neurological disabilities. The device’s innovative engineering, combined with ambitious visions for future human-AI integration, positions Neuralink at the forefront of neurotechnology. While challenges remain, the ongoing clinical evaluation in the UK is a critical step toward broader medical and potentially cognitive enhancements enabled by brain-computer interfaces.